Feasibility of intratumoral delivery
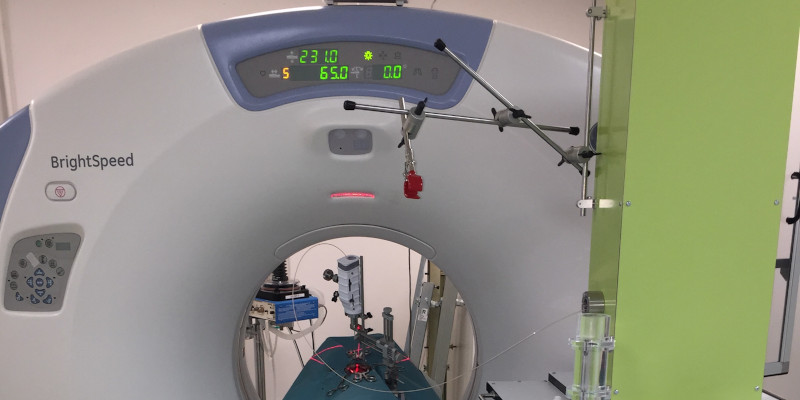
EVEON contributes to studies about feasibility of intratumoral delivery to induced glioblastoma, in a large animal model. EVEON developed the preclinical injection system for this project. Work on this subject has been published in the journal Plos One.
Abstract : Glioblastoma is the most aggressive primary brain tumor leading to death in most of patients. It comprises almost 50–55% of all gliomas with an incidence rate of 2–3 per 100,000. Despite its rarity, overall mortality of glioblastoma is comparable to the most frequent tumors. The current standard treatment combines surgical resection, radiotherapy and chemotherapy with temozolomide. In spite of this aggressive multimodality protocol, prognosis of glioblastoma is poor and the median survival remains about 12–14.5 months. In this regard, new therapeutic approaches should be developed to improve the life quality and survival time of the patient after the initial diagnosis. Before switching to clinical trials in humans, all innovative therapeutic methods must be studied first on a relevant animal model in preclinical settings. In this regard, we validated the feasibility of intratumoral delivery of a holmium (Ho) microparticle suspension to an induced U87 glioblastoma model. Among the different radioactive beta emitters, 166Ho emits high-energy β(-) radiation and low-energy γ radiation. β(-) radiation is an effective means for tumor destruction and γ rays are well suited for imaging (SPECT) and consequent dosimetry. In addition, the paramagnetic Ho nucleus is a good asset to perform MRI imaging. In this study, five minipigs, implanted with our glioblastoma model were used to test the injectability of 165Ho (stable) using a bespoke injector and needle. The suspension was produced in the form of Ho microparticles and injected inside the tumor by a technique known as microbrachytherapy using a stereotactic system. At the end of this trial, it was found that the 165Ho suspension can be injected successfully inside the tumor with absence or minimal traces of Ho reflux after the injections. This injection technique and the use of the 165Ho suspension needs to be further assessed with radioactive 166Ho in future studies.
EVEON developed the preclinical injection system for this project. The injector is located beside the operation table in CT room. The injection needle is connected to the injector through a connection tube to transfer the Ho suspension from the injector to the needle
Mehrdad Khoshnevis, Claude Carozzo, Richard Brown, Manuel Bardiès, Catherine Bonnefont-Rebeix, Sara Belluco, Christophe Nennig, Lionel Marcon, Olivier Tillement, Hélène Gehan, Cédric Louis, Ilyes Zahi, Thierry Buronfosse, Thierry Roger, Frédérique Ponce
Plos One, 18 June 2020
More details:
- Article published in 2020 on Plos One website: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0234772
- Article published in 2022 on Frontiers website https://www.frontiersin.org/articles/10.3389/fonc.2022.923679/full?

